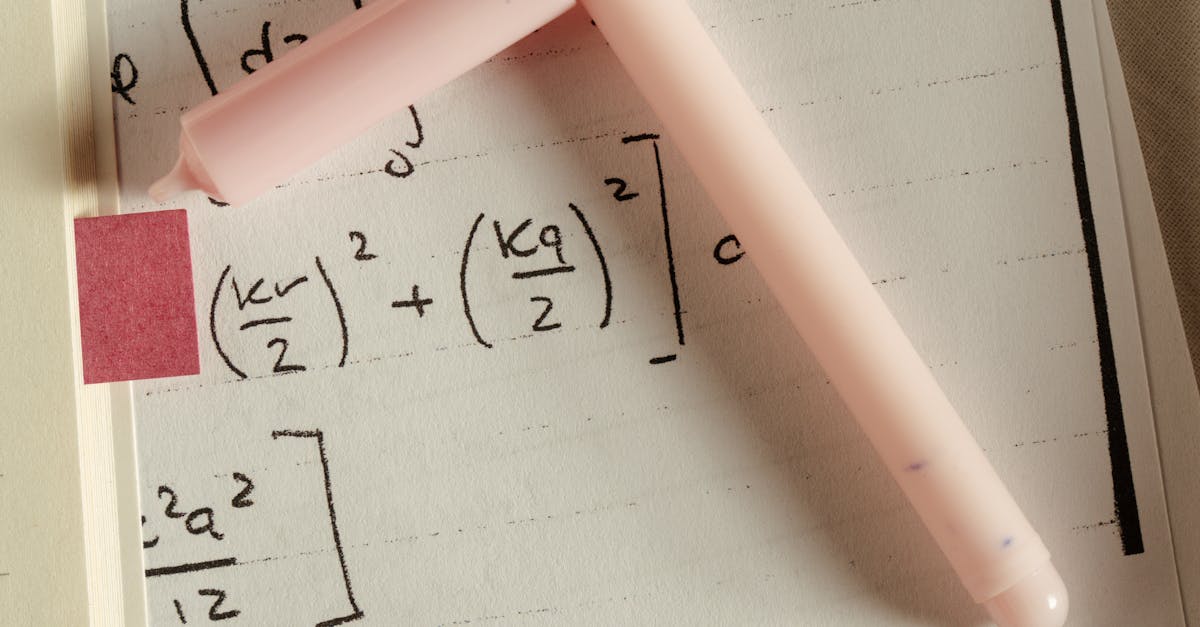
How to find empirical formula from percent composition by mass?
The chemical formulas of a pure chemical element are just representations of the atoms that make up the element. A chemical formulary is a list of the chemical elements and their chemical symbols, along with their chemical properties, chemical reactions, and atomic structures. There are more than 100 different chemical elements, each with their own unique properties.
How to find the empirical formula for percent composition by mass?
The first thing you do is to determine the most common element in your sample by mass. This is known as the major constituent. To do this, add up the total mass of all the elements in the sample and then divide this sum by the total mass of the sample. For example, say you have a sample of five different minerals. Their total mass is 27 grams. The total mass of all the minerals in the sample is 27 grams. Now add up the mass of all the minerals in the
How to find empirical formula from mass percent?
If you have a chemical reaction that converts one element into another, the empirical formula of the resulting product is the sum of the two atomic weights of the original atoms, divided by the sum of the atomic weights of the elements in the initial products (or the sum of the atomic weights of the products divided by the sum of the atomic weights of the reactants).
How to calculate empirical formula from mass percent?
First, find the atomic weight of the chemical element that makes up the mixture. The easiest way to do this is by using the periodic table. Next, look up the percentage of each element based on the overall mixture. The sum of all of the percentages should add up to 100%. Now you can use your empirical formula calculator to find the chemical equation of your mixture.
How to get empirical formula from mass percent?
The first thing you need to do is find the number of atoms in the chemical element, which can be found at the Periodic Table of the Elements website. The Periodic Table gives you the number of atoms for each chemical, and you can use this number as a starting point. For example, you can use the number of atoms for carbon to find an empirical formula for carbon dioxide.